Alfred Nobel stipulated in his will in 1895 that the prizes he bequeathed should be awarded to “those who, during the preceding year, have conferred the greatest benefit to humankind.” Some dismay was understandable, then, when the 2021 prize in physiology or medicine passed over the work that had, since the beginning of that year, already saved an estimated half a million lives worldwide by delivering vaccines against Covid-19.
The award committee’s deliberations apparently now happen too slowly to respect the letter of Nobel’s bequest. But Nobel recognition for the vaccines, developed astonishingly fast and displaying remarkable efficacy, will surely come soon, perhaps even this year. These jabs, which have made it possible at least to imagine an end to the pandemic, owe a great deal to the arrival of so-called mRNA technology for making medicines. The first Covid vaccines to receive emergency-use authorisation in December 2020—one made by Moderna, the other by German-based BioNTech together with Pfizer—used this new technique. And these vaccines have accelerated the introduction of a technology which could, its proponents say, fundamentally transform the way medicine and healthcare work.
The advent of mRNA is not the sole reason why the vaccines were developed with such unprecedented speed, less than a year after SARS-CoV-2 was discovered. Those that followed soon after, such as the Oxford AstraZeneca vaccine and those made in Russia and China, use a different approach. But the pandemic has established the scope, flexibility and efficacy of this new technology—the mRNA vaccines conferred an astonishing 92 to 95 per cent protection against the original virus (although new variants have reduced that margin). They were the culmination of several decades of research that came to fruition just when the world needed it—and as such, are testament to the importance of nurturing fundamental research long before applications are in sight.
“We and others in the field were already convinced that mRNA was the way to go to make highly effective vaccines,” says Melissa Moore, a chief scientific officer at Moderna, based in Cambridge, Massachusetts. “What Covid did was to accelerate the manufacturing process. No mRNA vaccine had been made at large scale before, and the pandemic really accelerated that in a way that wouldn’t have happened otherwise.”
Now Moore and many others are convinced that mRNA technology will change the way we make medicines more generally, offering potential benefits for treating not just infectious diseases but also major killers such as cancers and heart disease. Microbiologist Norbert Pardi of the University of Pennsylvania is confident we will see improved influenza and malaria vaccines, and that “we will be able to develop brand new vaccines against pathogens that we cannot efficiently target with traditional methods.”
All these possibilities arise because mRNA treatments are a new kind of medical intervention. In contrast to drugs that have a specified molecular target in the body, mRNA-based medicines trigger our bodies to find their own solution against pathogens or illness within the versatile and powerful resources of the immune system. And while that is in a sense the principle of all vaccines, those made with mRNA can be rapidly developed, reconfigured and combined. Medicines that make use of mRNA are what drug designers call a platform technology: a method with multiple, readily accessible uses. The big question is whether our own body’s ingenuity to cure and protect via the immune response will prove the equal of nature’s ability to adapt and evade.
Do it yourself
The human capacity to survive (most of the time) a regular onslaught of viruses and other pathogens is testament to the resourcefulness of our immune system. Vaccines prepare the body’s immune response so that it can quickly and effectively fight off such threats. Viruses are the most stripped-down biological agents imaginable: barely alive at all in isolation, they contain viral genes (encoded in a DNA molecule or, in the case of SARS-CoV-2 and many others, in the closely related molecule RNA) packaged in a protein coat. The viral proteins latch onto some of the proteins on the surface of our cells, enabling the virus to penetrate inside and hijack the molecular machinery. The infected cells are forced to copy the viral genome—and that is how the virus replicates.
The job of the immune system is to recognise particles in the body that are foreign to it—“antigens,” such as parts of pathogenic viruses—and then marshal specialised white blood cells to combat the incursion. Some of those are called B cells, which produce antibody proteins that can attach to a virus, either blocking its action or tagging it for destruction by so-called T cells. A vaccine primes the immune system in advance by introducing it to a harmless fragment of the pathogen, typically a small piece of one of its proteins: for SARS-CoV-2 vaccines this is generally the “spike protein” (or a piece thereof) that sticks to human cells. Some B and T cells will remember the antigen so that the immune system can quickly produce the right antibodies if it is encountered again. Primed thus, the immune system can instantly respond to and suppress the virus that a vaccine has trained it to recognise.
Some vaccines deliver the antigenic protein itself, such as that developed by the US biotech company Novavax, which was recommended for use by the World Health Organisation (WHO) last December and which also includes a substance called an adjuvant that stimulates the immune system. Others carry the genetic information needed for the body to make the protein encoded in a harmless virus—in the case of the AstraZeneca vaccine, the carrier is a disabled common-cold adenovirus. Some conventional vaccines use an inactivated form of the full virus they protect against, which the immune system can learn to recognise (in this case the genetic material of the virus is either removed altogether to prevent replication, or modified to weaken it). But producing a genetically modified or inactivated virus or a purified viral protein takes time and effort.
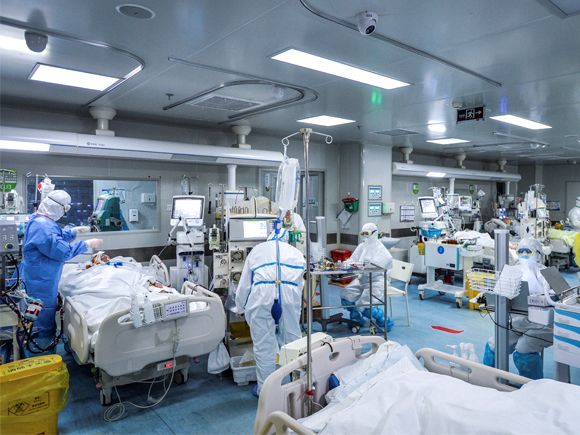
An mRNA vaccine avoids those laborious steps by providing our cells with the instruction to make a piece of the spike protein themselves, but without having to insert that instruction into another virus—instead, it is encoded in a strand of RNA. Our own cells make RNA all the time: it is the intermediary that enables a gene in our DNA to build a corresponsing protein (the “m” stands for messenger; other types of RNA are made in cells too). Any given gene has a specific sequence of the chemical building blocks of DNA; when the gene is active, this sequence is copied by enzymes into the equivalent sequence in an mRNA molecule, and that sequence (the “message”) is read by a complicated piece of molecular machinery and used to build the respective protein molecule. An mRNA vaccine delivers the message encoding an antigenic protein, inside a tiny capsule made from lipid (fatty) molecules. Our cells set about making the protein, triggering the immune system to produce antibodies that in future will be capable of recognising it and sounding the alarm. “All we are doing is tapping into basic biology that is already happening in the body,” says Moore.
The machinery to make mRNA vaccines is no bigger than a washing machine
Because an mRNA vaccine induces your body to make the protein itself, she adds, “it is very much mimicking a viral infection, where the body is making the viral proteins. By tapping into all those pathways, mRNA vaccines give a strong B and T cell response.”
The beauty is that all you need to know to make an mRNA vaccine is which sequence the piece of mRNA requires to make an antigenic protein: a straightforward thing to deduce with today’s sequencing technologies, once the virus has been isolated. The sequence of the SARS-CoV-2 genome was deduced, and announced worldwide, within days of the virus itself being identified in China in January 2020.
Which part of that sequence to encode in an mRNA vaccine is not necessarily obvious. But fortunately, a lot was already known about coronaviruses: the Covid virus is closely related to the SARS virus that caused around 800 deaths globally in 2002–2004 (whence the name). Researchers knew that these viruses have spike proteins that enable them to infect human cells, and so they quickly identified the sequence of the Covid-19 spike.
“We went from the sequence of the SARS-CoV-2 virus to having an mRNA vaccine ready to go into people’s arms for the phase one trial in 45 days,” says Moore. What limited the speed of getting a vaccine ready for use wasn’t the basic science of making one, but the thorough clinical testing needed to establish safety and efficacy before administering billions of shots worldwide. In principle, these vaccines can be quickly retooled to deal with new variants that have mutations in their sequences. Such mutations arise by chance during viral replication; if they confer some advantage on how readily a mutant form can infect cells or evade the immune system, that variant will spread rapidly by Darwinian selection—especially if high infection rates create the opportunity.
“It is very easy to modify mRNA vaccines, making them ideal to target pathogens that change very quickly,” says Pardi. “This is not true for the traditional vaccines.” Such modified vaccines probably wouldn’t need a repeat of full clinical trials before use. In practice, whether or not it would be worth doing this depends on how much loss of protection a new variant induces, balanced against the huge costs and logistical challenges of retooling the manufacturing and launching a new round of vaccination. Moderna has just developed a vaccine against Omicron, which it says is able to elicit a good immune response against the fast-spreading newer BA.4 and BA.5 subvariants. The company hopes it will be approved as a booster for use in the autumn. Pfizer and Novavax are also testing vaccines that specifically target Omicron.
The road to mRNA
The development of mRNA vaccines didn’t happen overnight. “There have been decades of research in basic science on these vaccines,” says Akiko Iwasaki, an immunologist at Yale. In fact, the earliest mRNA-based vaccine, developed for flu in mice, was tested in 1993, although the current approach of packaging the RNA in lipid nanoparticles wasn’t well developed until the early 2010s. So it’s fortunate but no accident, Iwasaki says, that they were ready just in time for the Covid pandemic.
In the early days of the research it was widely thought that mRNA was too expensive and chemically unstable to provide a viable vaccine. It was only from around 2010 that opinion changed—thanks in part to a discovery, by Katalin Karikó and Drew Weissman at the University of Pennsylvania in 2005, of how to modify chemically the mRNA so that it doesn’t fall foul of the body’s immune defences. (Moderna, which uses that approach, is named after such “modified RNA.”) Karikó and Weissman are among the names often now touted for a Nobel, along with husband and wife Uur ahin and Özlem Türeci of BioNTech and Sarah Gilbert at Oxford. (In truth, singling out no more than three individuals for recognition of a breakthrough that required enormous co-ordinated effort would make little sense.)
But although those researching mRNA vaccines were confident they could work, no one could be sure precisely how effective they would be. When the results of the final, large-scale phase three clinical trials came in, “there were stratospheric rates of vaccine efficacy compared to more traditional technologies,” says Elad Sharon of the US National Cancer Institute. “When we got the phase three results of 95 per cent efficiency, we were a mixture of relieved, excited and amazed,” says Moore. “It worked better than all expectations.”
There are other advantages. “One of the things that’s really exciting about mRNA vaccines is the small footprint of the manufacturing equipment required,” says Moore. Traditional vaccines typically require huge bioreactors, whereas the machinery to make mRNA vaccines “is no bigger than a large washer-dryer machine in your house.” Moore says Moderna is now setting up local vaccine manufacturing facilities all over the world and working with Darpa, the research branch of the US Department of Defense, “to miniaturise the entire process so that it could be put into a single shipping container and sent anywhere in the world, to rapidly respond to emerging diseases.”
There are challenges too, though. One, much-discussed during the pandemic, is that the mRNA vaccines made so far need to be stored at very low temperatures to stay stable: the Pfizer mRNA vaccine must be kept below -70°C. The AstraZeneca vaccine, in contrast, can be shipped at normal temperatures and stays stable for several months in an ordinary fridge. “This is a significant issue in many countries where the infrastructure is poor and storage of mRNA vaccines at low or ultra-low temperature is not possible,” says Pardi. But Moore is confident that the intense research now invested in this field will crack the problem.
What’s more, the new technology tends to be more expensive than traditional methods: currently, the Moderna vaccine costs about $20 a shot, substantially more than the AstraZeneca jab at $2–$5. But Pardi believes the cost will come down “once companies optimise the manufacturing processes and the cost of vaccine components decreases.”
Prevention and cure
Now that the potential of mRNA-based medicines has been demonstrated, what’s next? In terms of infectious disease, influenza is a key target: according to estimates by the WHO, there are 290,000–650,000 flu-related deaths globally each year. There are several influenza viruses and they mutate fast, so that each flu season’s vaccine needs to be tailored to the variants predicted to be the most likely threats. Not only do mRNA vaccines offer the prospect of easy updates for new strains, they also create the possibility of packaging several different mRNA molecules into a single shot to protect against several strains—or indeed, several viruses. Work is currently underway on a “combo vaccine” that would protect against both flu and SARS-CoV-2, as well as another dangerous pathogen called respiratory syncytial virus. “We are hoping that we can get to a single booster shot that people take either once a year or once every six months, to immunise them against those three major viruses,” says Moore.
One of Moderna’s mRNA vaccines, which was already at an advanced stage when the Covid pandemic broke out, acts against cytomegalovirus (a herpes virus) and is now in phase three clinical trials. The company is also developing a vaccine against HIV—which has been resistant to previous efforts because it mutates so fast. “Many have tried, but we think we have a new way of training the immune system to recognise parts of the HIV virus that can’t be easily mutated,” says Moore. Still at phase one trials, the treatment would involve a three-part vaccination programme that administers slightly different versions of the vaccine each time. BioNTech, meanwhile, have teamed up again with Pfizer to develop an mRNA vaccine against the painful viral condition of shingles.
It’s not just about infectious diseases. One of the earliest tests of an mRNA therapy in 1995 was for cancer, and this was a major focus of research before the pandemic. Cancer cells are not exactly pathogens, but regular body cells that have acquired genetic mutations which alter or disable some key proteins and unleash rapid proliferation. If the immune system can be trained to recognise those mutated proteins, it can be induced to attack cancer cells specifically.
“You’re trying to alert the immune system to an aberrant protein that’s a marker of cancer activity,” says Sharon. But because “most of the proteins a cancer cell has are derived from normal proteins, whereas a viral protein is entirely foreign, the cancer proteins are likely to be less immunogenic” (that is, likely to provoke a weaker immune response). The challenge is to supercharge the immune system so that it can recognise and treat those proteins as a threat.
Efforts to develop cancer vaccines using traditional approaches have been going on for years, but with very limited success. A treatment called Provenge has been approved for therapeutic use (against advanced prostate cancer) in the US and UK, but Sharon explains that “it has not been terribly popular because it gives only a small survival advantage to people with prostate cancer, is complicated to administer, and is marketed quite expensively.” (Dendreon, the manufacturer, says its product helps extend the lives of patients.)
Whether mRNA therapies can overcome the shortcomings remains to be seen. But they should make some new options possible. One approach being pursued seeks to create a vaccine that, while triggering an immune response with a given antigen, will also put the immune system on more general alert. A potential advantage of doing this, Sharon says, is that the response, although initiated by a particular cancer-related molecule, might constantly evolve alongside a mutating cancer itself. “Even if you’re telling the immune system to hit target X, those activated immune cells might then see something else too that it thinks is a problem, and respond to X plus something else,” he says. The problem of tumours mutating rapidly and evading the immune system is partly what has stymied vaccine attempts previously.
HIV has been resistant to vaccine efforts because it mutates so fast
Another opportunity is that, because of the ease of tinkering with mRNA vaccines, cancer vaccines could be tailored to the individual. Regular biopsies of a tumour could be used to update a treatment so that it is always hitting the latest version of that person’s cancer. And because the medicine is being given to just one person, there would be no need for (or point in) large clinical trials of that personalised treatment before regulatory approval. Cancer vaccines might even be administered not to treat an existing tumour, but preventatively: given to people known to have a genetic vulnerability to certain types of cancer, to prepare their immune system for action should that threat manifest itself.
Another big target is heart disease. Moderna, in partnership with AstraZeneca, is currently conducting late-stage phase two clinical trials of an mRNA medicine which encodes a protein triggering the growth of new blood vessels. Moore says that injecting the packaged mRNA directly into the heart muscle of patients who have had heart attacks due to blocked arteries could aid repair of the damaged tissue.
Metabolic diseases could also be treated using mRNA. These conditions commonly arise from genetic mutations that prevent the body from producing some enzyme needed for normal metabolic processes: for example, the rare inherited condition called phenylketonuria occurs when the body is unable to break down a certain amino acid, potentially leading to brain damage. “Those diseases by and large can’t be addressed by making the protein outside the body and delivering it by injection,” says Moore, “because a lot of the metabolic diseases require that the missing protein work inside the patient’s cells”—and a protein injected into the bloodstream generally can’t get inside. By providing the resources for cells to make their own protein in situ, an mRNA medicine could overcome that obstacle. As is the case with insulin to treat diabetes, the treatment would require regular dosage, perhaps every two weeks or so.
It all sounds rosy, but no one thinks mRNA medicines are a panacea. They won’t work against all pathogens, for instance. Some existing vaccines against bacterial infections don’t even use proteins as the target antigens, but instead prime the immune system using sugar molecules that appear on the surface of the bacterial cell—which can’t be encoded in mRNA.
Some viruses too are tougher opponents. “Just because we were successful for SARS-CoV-2 doesn’t mean we are going to be successful for others, if they have many capabilities for mutation and evasion,” says Iwasaki. Herpes viruses (on which she has worked for years) tend to excel at evading the immune system and are hard targets for a vaccine. And if the next pandemic is caused by a virus we know little about, basic research will be needed before we even know what to target. “We were lucky with SARS-CoV-2, because we had experience with other coronaviruses and therefore we knew we wanted to target the spike protein,” says Pardi. “The problem is that there are many pathogens… viruses, bacteria, fungi and so on, that are really complex and for which it is hard to identify good vaccine targets.” That’s why, Moore says, “I cannot emphasise enough how much basic curiosity-driven research on infectious agents by folks working [in] universities and government labs is crucial.”
All the same, mRNA technology “is revolutionising vaccinology,” says Beate Kampmann, director of the Vaccine Research Centre at the London School of Hygiene and Tropical Medicine. Indeed, it is shifting the narrative of how we design and make medicines in general. Steven Soderbergh’s 2011 medical thriller Contagion was credited by former health secretary Matt Hancock with prompting him to order large quantities of vaccines while they were still in development. But the vaccine technology it depicted is now outmoded, says Iwasaki. “If Hollywood were to make that movie now, they’d be using mRNA.”












